Application of CH4 Technology on the Synthesis of Methanol
Yingzi Kang
Published Date: 2018-10-09Yingzi Kang*
Number 4 Middle School, Daqing, 163000, China
- Corresponding Author:
- Yingzi Kang
Number 4 Middle School, Daqing, 163000, China
Tel: +864595168505
E-mail: hailingma@yeah.net
Received: September 28, 2018; Accepted: September 29, 2018; Published: October 09, 2018
Citation: Kang Y (2018) Application of CH4 Technology on the Synthesis of Methanol. Chem Inform Vol. 4 No.2:3.
Abstract
Methanol has always been the basic raw material for new energy sources. In addition, it is an important kind of clean energy. The carbon and oxygen resources inside carbon dioxide are used to synthesize methanol through hydrogenation process, which helps to realize the recycling of carbon resources and reduce the dependence on fossil energy and the burden of environment. This project focuses on the catalyst in the process of the synthesis of methanol and the use of kinetic models for analysis to prove the accuracy of the process of synthesis of methanol.
Keywords
Methanol; Carbon dioxide; Hydrogenation; Catalyst; Kinetic model
Introduction
Methanol is one of the most important basic materials in chemical industry, mainly for the production of chemical products such as formaldehyde, two ether, acetic acid and some chemical fuel such as olefins (ethylene and propylene), aromatics (benzene, toluene and xylene), gasoline, in order to lessen the dependence on oil resources [1-7]. In addition, methanol is a clean energy source that can be used as fuel for internal combustion engines or fuel cells. The carbon and oxygen resources of carbon dioxide are used to synthesize methanol through hydrogenation process, which helps realize the recycling of carbon resources and reduce the dependence on fossil energy and resources to reduce the environmental burden [8-17].
Energy problem has become a strategic issue that restricts the development of China’s national economy [18-20]. From the perspective of national security, the stable supply of energy resources is always the focus of attention of one country’s dependence on imports and the core content of national security. With the accelerated process of industrialization and urbanization in China and the upgrading of the consumption structure of the residents, the clean and efficient energy, such as oil and natural gas, will occupy an increasingly important position in the future. At present, China’s oil consumption is heavily dependent on imports. Oil resources have been closely linked with national security and become the core of China’s energy security strategy [21-25].
In China’s energy reserves, coal accounts for 94%, oil accounts for 5.4% and natural gas only accounts for 0.6%. The energy structure characteristics of this kind of “rich coal and less gas” determines that China’s energy production and consumption based on coal will be dominated in the long term. With the sustainable development of the national economy, the demand for energy products, especially clean energy continues to grow. Based on the current situation of China’s special energy structure, the development of coal-based energy and chemical industry has become the main way to solve the problem of energy [26-30]. Polygeneration system based on coal gasification, facing the energy demand of liquid fuel shortage, serious environmental pollution and a series of problems, has been a solution to the sustainable development of China’s energy sector. Coal gasification into synthesis gas after purification can be used in the production of chemical raw materials, liquid fuel (synthetic oil, methanol, dimethyl ether) and electric. The liquid fuel produced by the multi co production system, in particular, methanol and two dimethyl ether can be used as a substitute for coal-based vehicle, which can partially alleviate the shortage of petroleum in our country. At the same time, methanol can also be used to produce olefins and propylene, which the coal chemical products alternative part of the traditional petroleum and chemical products, has the great significance of reducing the consumption of oil.
Methanol is an important chemical raw material but also a potential fuel for vehicle fuel and fuel cell. Therefore, the research and exploration of the synthesis of methanol has been paid more attention in the world. Especially in recent years, with the emergence of the energy crisis, C1 chemical has been developed as important material of methanol [31]. The methanol industry has been developing rapidly in the world. In the basic organic chemical raw materials, the amount of methanol ranks fourth, only after ethylene, propylene and benzene [32-38].
As a basic chemical raw material, methanol has a wide range of uses in the chemical, pharmaceutical, textile and some other fields. Methanol is mainly used for the manufacture of formaldehyde, chloride, acetate, methyl amine, methyl methacrylate, methyl formate (MF), dimethyl ether (DME), dimethyl carbonate (DMC), dimethyl terephthalate (DMT), methyl tert-butyl ether (MTBE) and a series of organic chemical products [39-41]. With the deep processing of methanol and increasing application of chemical products, methanol has broad application prospects in such areas:
• Methanol has a high-octane number and oxygen-enriched fuel. The combustion process sufficiently thorough than gasoline exhaust CO, SO2, NO2 and hydrocarbons can be significantly reduced, as a clean fuel to replace gasoline or blended with gasoline use;
• The methanol fuel cell will be put into commercial operation;
• Methanol as a preliminary cracking feedstock use in PSA hydrogen production;
• Preparation of methanol microbial protein feed as well as food additives, abroad industrial unit;
• Methyl formate (MF), dimethyl ether (DME) have become the hot products in chemical Cl;
• Methanol to gasoline (MTG), methanol to olefins (MTO), methanol to propylene (MTP) technology breakthroughs have become an important way to coal gas and coal to olefins.
Materials: The Choice and Research of Catalyst
Catalyst composition
Active component: According to the different active components, the catalyst for CO2 hydrogenation to methanol synthesis can be roughly divided into two categories. One kind is the copper base catalyst which takes the copper element as the main active component, and the other is the supported catalyst with noble metal as the active component.
Copper based catalyst: Most of copper-based catalysts for CO2 hydrogenation were developed on the basis of methanol synthesis from syngas. Although since 1960s, ICI company as Cu/ZnO/Al2O3 catalyst on behalf of production has become a commercial catalyst for methanol synthesis gas, but the activity center, especially the Cu state in the catalytic reaction exists a lot of controversy [42-45]. When copper-based catalysts are used for CO2 hydrogenation, the problem becomes more complex because of the oxidation of CO2 gas.
Some researchers think that the copper-based catalysts in the synthesis of methanol in the presence of Cu0 and Cu+ (or Cu delta +) in the form of CO2 and these two forms of Cu species are active center. This basis comes from two aspects of experimental detection and theoretical calculation. Cu+ detection is generally used XPS technology. Because the metal Cu and Cu2O bonding energy (Cu 2p3/2 were 932.6 eV and 932.4 eV) are very close, Cu0 and Cu+ are unable to distinguish. Cu2O and metal Cu and Auger spectra of CuKLL line difference (918.65 eV and 917.9 eV), Cu0 and Cu+ can be distinguished. Therefore, XPS need to be used with the Auger spectrum. Stoczynski [4] used Cu/ZnO/ZrO2 catalyst for CO2 hydrogenation reaction to check the catalyst after 10 days and found the presence of Cu2O. Toyir [5,6] prepared using Cu/ZnO/SiO2 as a precursor of zinc oxide and zinc, and the presence of Cu+ was detected in the reaction. They found that Cu+ has improved the selectivity of methanol, and the ratio of Cu+/Cu0 could be adjusted by changing the amount of Ga added. Saito [7] also proposed a similar point of view and thought the catalysts were in the best performance when the ratio of Cu+/Cu0 is 0.7. Xu Zheng [8,9] studied CO2 hydrogenation over CuO-ZnO based on catalysts and pointed that the active center of CuOZnO solid solution of Cu- - -Zn-O (- oxygen hole), Cu valence in activity center are Cu+ and Cu0. Other techniques were also used to detect the presence of Cu+ in Cu based on catalysts. Fierro [10] used H2-CO2-H2 redox cycle to confirm that reduction of Cu in the CuO/ZnO can be partially oxidized by CO2, which shows that there is Cu delta +, and pure Cu in the CuO does not exist in this case. EXAFS study showed that Cu/ZrO2 in the catalytic hydrogenation reaction of 76% Cu0 was CO2 oxidation, which Cu2+ accounted for 27% and Cu+ accounted for 49% [11]. Arena [12] used CO as molecular probe molecules formed by infrared spectroscopy to the Cu/ZnO/ZrO2 catalyst Cu 8 + Cu + 8. They pointed out that the metal oxide interface is located on the interaction between Cu and ZnO, ZrO2 to Cu 8 + stable. In addition, density functional theory calculations also show that Cu + delta appears in the Cu/ metal oxide interface. The formation of methanol reaction mainly in Cu + delta, and reverse water gas reaction is carried out in Cu0 [13]. Wang [14] used Unity Bond Index-Quadratic Exponential Potential method to Cu (100) on the CO2 hydrogenation reaction which carried out the theoretical calculation. The results showed that the relationship between the coverage degree of oxygen and the reaction properties of the copper crystal surface shows a volcanic curve, and the ratio of Cu+/Cu0 controls the activity of the catalytic reaction [46-48].
The classifying of catalyst for industrial methanol catalysts and its advantages and disadvantages
Zinc-chrome catalyst: Zinc-Chrome (ZnO/Cr2O3) Catalyst is a kind of high-pressure solid catalyst, which was developed for the first time by BASF from Germany in1923. Zinc-Chrome Catalyst has low activity, for getting higher activity, the operating temperature must keep temperature between 590 k to 670 k. or getting higher conversion ratio, the operating pressure must keep between 25 Mpa to 35 Mpa, therefore, it is named high pressure catalyst. The characteristics of Zinc-Chrome Catalysts are including: a) Extremely efficient in preserving heat. It can support overheating process whose temperature difference above 100°C. b) Insensitivity to sulfur. c) High mechanical strength. d) Long service life, wide use range, easy operating control e) Compared with copper-based catalyst, it has low activity, low selectivity, hard to rectification (complex impurity in product). Due to the fact that the mass fraction of Cr2O3 in this kind of catalyst is up to 10%, it has become one of the most serious pollution sources.
Copper-based catalyst: Copper-based whose major components are CuO, ZnO and Al2O3(Cu-Zn-Al) is a low temperature and low-pressure methanol synthesis catalyst. It was developed by ICI from England and Lurgi from Germany [49]. The operating temperature of the copper base catalyst for low (middle) pressure is between 210℃ to 300℃, the pressure is between 5 Mpa to 10 Mpa. Compared with the traditional synthetic process, this kind of operating temperature is much lower, and it’s good for methanol reaction equilibrium. The characteristics of Copper-based catalyst are including:
• High activity, the conversion per pass is between 7% to 8%;
• High selectivity, it can be up to 99%, there’s only a little CH4, CH3OCH3, HCOOCH3 in the impurity, so it’s easy to get the high purity of methyl alcohol;
• Poor resistance to high temperature and sensitive to sulfur.
Palladium-based catalyst: Because the selectivity of Copperbased catalyst is over 99%, the research direction of the new type catalyst is to further improve the catalyst’s activity and thermostability and prolong the service life of catalyst. Most of the new type catalysts are based on transition metals, noble metals and so on. However, compared with traditional catalyst, its activity isn’t ideal. Such as some kinds of noble metals, the increase of its activity isn’t evident, even tends to reduce.
Molybdenum-based catalyst: The Copper-based catalyst is an important catalyst in synthesis of industrial methanol, however, due to there’s a little H2S, CS, Cl2 and so on in the feed gas, leading to catalyst poisoning. Therefore, it has become more and more popular to research sulphur-resistant catalyst. Zhang Jiyan from Tianjin University researched MoS2/K2CO3/MgO-SiO2 sulphur methanol synthesis catalyst, the temperature is 533 K, the pressure is 8.1 Mpa, the airspeed is 3000 h-1, φ(H2): φ(CO)=1.42, the sulphur concentration is 1350 mg/L, CO conversion is 36.1%, the selectivity of methanol is 53.25%. Though this kind of catalyst has higher conversion per pass, its selectivity is only about 50%. Besides, there’s still a large gap in the industrial application because it’s by product is complex.
Besides, there’s Cu-Zn-Al-V series of catalysts. Cu-Zn-Al-V copper-based catalyst are including C207、C301、C3011 、NC5011、C306 and C307 in South of research group, CN J202 、C302、C3021、C3022、CN J206 and XNC98 in Southwest chemical of research group.
Copper-based catalysts are widely used at present. The research direction of copper-based catalyst will be low temperature, low pressure, high activity, high selectivity, saving energy and protecting environment in the future.
Catalyst components functions
Catalyst components’ functions are including:
CuO: CuO will become the active centre of the catalyst after revivification, which exists in the active interface of the CuO-Cu.
ZnO: ZnO’s functions including: stabilize the active centre- Cu+ (Cuprous), keep Cu (Cu powders) high dispersion, activate H2 (increase the rate of catalyst of hydrogen sorption), absorb the toxicant in the synthesis gas. (exists by ZnX after absorbing). Cu/ ZnO interface form the unique active centre. (Some theory came up with that the reaction is double function, CU and ZnO are involved in different steps to form a general mechanism.) ZnO makes Cu’s special bath crystal or surface defects stable.
Al2O3: Al2O3 hasn’t catalytic activity for the synthesis reaction, but when the Al2O3’s content is about 10%, the crystal size of the catalyst is reduced, the radio of the copper surface and the catalyst of the total surface is maximum, and the activity is best. In additional, it can improve catalytic stability (Al2O3 can be formed as a dispersing agent and the separation agent of aluminium acid and zinc to prevent the sintering of copper particles, which is a stabilizer of high dispersion Cu/ ZnO.).
Graphite: Add graphite after baking, to facilitate the forming of punching and pressing, with a sense of lustre.
Changes in catalyst
Deactivation of the catalyst: The activity of the catalyst is one of the key factors to determine the new technology of the methanol synthesis will be successful or not. During the process of methanol production, there always appears catalyst poisoning, high temperature hardens and so on which belongs to abnormal phenomenon. These abnormal phenomena not only shorten the service life of methanol synthesis catalyst, but also influence the quality of methanol, such as heat inactivation, carbon deposition, deactivation and inactivation, pollution, intensity, intensity decreased and so on.
Catalyst poisoning: Methanol-Synthase Cu-Katalysatoren is extremely sensitive to sulphur, the active component Cu and S in catalyst are transformed into copper sulfide and loss activity after poisoning. This process is named surface poisoning, which belongs to Flow- Solid uncatalyzed reaction. Durting the catalyst with surface poisoning was used, reaction components need to reach active surface through the deactivated surface. This phenomenon is serious for outer diffusion. The study showed that the copper in the fresh catalyst was in the form of highly dispersed CuO, and the zinc existed in the form of highly dispersed ZnO2. However, there’s a little CuS and ZnS in the deactivated catalyst. At the same time, the diffraction peak of copper is sharp, the deactivated catalyst of the Cu crystals is (6.0-8.5) *10- 9m. This phenomenon shows sulphur poisoning during process. In the process of industrial production, sulphur and nickel in raw gas is one of the reasons for deactivation of methanol catalyst. Industrial production also shows that the fine desulfurization of raw gas can effectively prolong the life of the catalyst.
Regeneration of the catalyst by H2O, CO, CO2, H2, NH3, H2O2, HNO3, and carbon dioxide, ether supercritical washing method for regeneration of the deactivated catalyst, the deactivation of the catalyst specific surface area increased, but also has a certain degree of increasing catalytic activity. Close to the activity of catalyst and the laboratory preparation of new catalysts were also prepared by acid leaching, this method is so good for regenerating a catalyst for methanol, and the preparation technology and further research is needed.
The improvement of catalyst
The research shows that the best ratio is 3:2(w), improve carrier alkaline and add L auxiliaries make Cu enrich to catalyst surface and improve the ratio of Cu/Zn surface. At the same time, adding La can also improve catalyst’s stability.
Methods
Theoretical basis of dynamics
Experimental flow shown in Figure 1 At the beginning of the experiment, the device was purged with nitrogen to remove the impurity and residual air of the device, and then the reducing gas was introduced into the reductive gas to carry out the temperature reduction and then the pre-prepared raw material gas was transferred to the kinetic data / RTI and gt; From the cylinder out of the gas first through the valve control pressure, and then control the flow through the mass flowmeter, and then through the deaerator to remove trace oxygen into the internal loop without gradient reactor, the catalytic reaction to produce methanol. After the reaction of high pressure gas pressure back to the pressure valve to atmospheric pressure, through the insulation pipe to the condenser, the condensation reaction of the mixture, and then by gas-liquid separator, the liquid product can be used for analysis, the Gas is divided into two, all the way through the gas chromatograph analysis of its composition, the other way through the soap film flow measurement flow after the emptying.
The experimental conditions were as follows: reaction temperature 483.15-523.15 K, pressure 5 MPa, loading C306 cylindrical catalyst (95 mm × 5 mm) 2 (mass ratio of catalyst to catalyst) 0.0369 g, filled with the same size of the glass beads and catalyst phase arrangement.
Dynamic model
The raw gas containing CO, CO2, H2, N2, CH4 was under the action of Cu based catalyst.
 (1)
(1)
 (2)
(2)
In the model, the reaction rate constant K and the adsorption constant K are expressed as follows:
 (3)
(3)
 (4)
(4)
Equation in:
 (5)
(5)
Kf1 ,Kf2 , the equilibrium constants of the reaction 1 and 2 are expressed in the degree of ease, and the ease of each component is calculated by the SHBWR equation of state.
The main reactions of methanol synthesis on catalyst are:
• CO+2H2=CH3OH
• CO2+3H2=CH3OH+H2O
• CO2+H2=CO+H2O
Selection of CO2, CO hydrogenation synthesis of methanol as an independent reaction, the kinetic equation of the Langmuir- Hinshelwood type, the reaction rate to the gas phase of the components of the ease to express.
 (6)
(6)
XRD patterns of selected Cu, Zn (Al) precursors: unaged sample(a), binary sample(b) and ex-LDH sample(c). The reflex marked with a star in (a) can be assigned to grease which was used for specimen preparation. Crystalline phases were identified as malachite ICDD 72-75 (black bars in c). The precursor of the unaged sample is amorphous, that of the binary catalyst contains a minor.
Fraction of aurichalcite in addition to the major phase zincian malachite, which the LDH precursor is phase pure according to XRD.
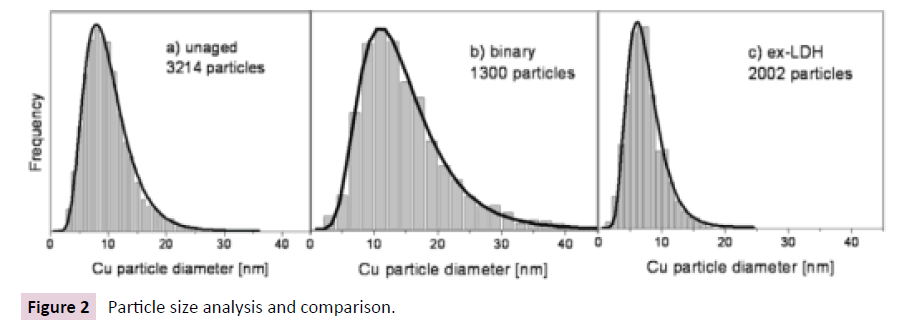
In Figure 2 Particle size distribution and long-normal fits for selected Cu\ZnO (Al2O3) catalysts: unaged sample(a), binary sample(b) and ex-LDH sample(c).
In Figure 3 Metal composition as determined with several local EDX measurements of selected Cu/ZnO (Al2O3) catalysts: unaged sample, binary sample and ex-LDH sample. Catalysts prepared from crystalline precursors (LDH and zincian malachite) exhibit a very narrow composition range. The catalyst from the unaged amorphous is less homogeneous, but still show significantly less scattering of the local composition compared to industrial catalysts (cf. data shown in Figure 4).
Graphical representation of the Rietveld fits for selected catalyst samples and the pure Cu reference material. The quality of the fit is representative for all patterns. The characteristic under-estimation of the intensity near the maximum of the 200 peaks at ca.42° 2θ in the pattern of the catalyst sample can be explained with the presence of twin boundaries and stacking faults leading to a broadening of this reflection [50].
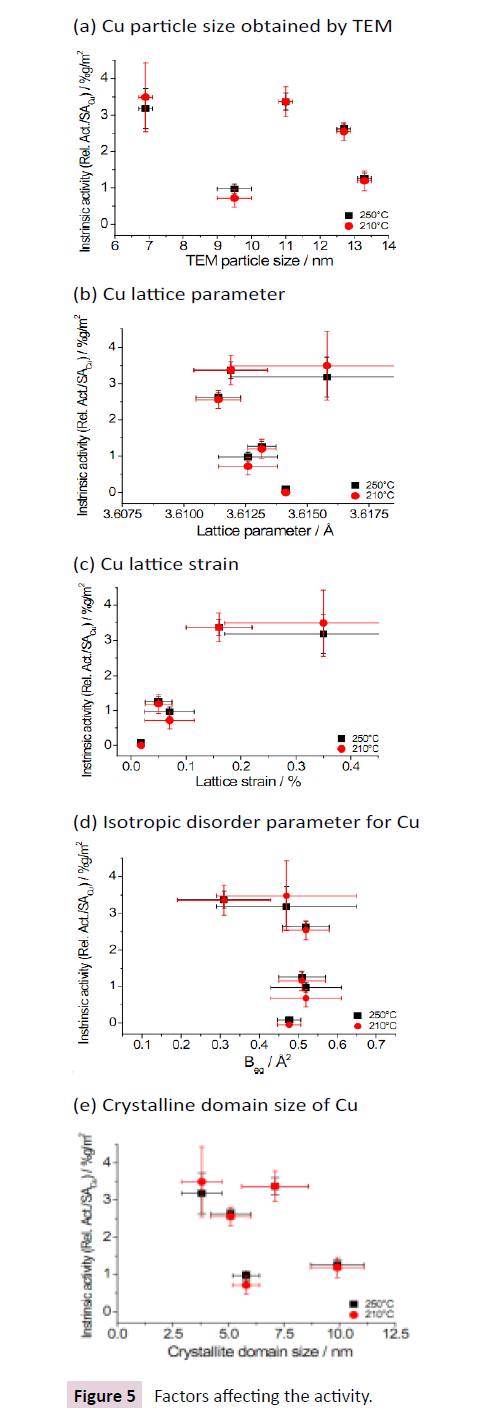
In Figure 5 Attempts to correlate the intrinsic catalytic activities of the Cu/ZnO/Al2O3 catalysts with the results obtained by TEM and Rietveld refinement is depicted.
• Cu particle size obtained by TEM;
• Cu lattice parameter;
• Cu lattice strain;
• Isotropic disorder parameter for Cu;
• Crystalline domain size of Cu.
In Figure 6 it is shown that Devolution of the Zn3p (blue), Cu3p (red) andAl2p (purple) contributions to XP spectrum of the reduced catalyst conv. The fit quality is representative for all spectra.
In Table 1, FHI sample database codes for selected sample are given.
| Sample | Precursor Sample Number | Calcined Sample Number | Reduced+ passivated Sample Number | Reduced Sample Number |
|---|---|---|---|---|
| Cu Ref (Cu) | 8052 | 8053 | 8535 | 8389 |
| Binary (Cu, Zn) | 7399 | 7400 | 8538 | 8387 |
| Ex-LDH (Cu, Zn, Al) | 7965 | 7988 | 8537 | 8386 |
| Unaged (Cu, Zn, Al) | 6980 | 7088 | 8536 | 8388 |
Table 1 FHI sample database codes for selected sample.
In this paper, the pressure is 5 MPa, the temperature is 483.15-523.15 K, the different raw material gas is composed of the condition, and the experiment has measured the copper base methanol
Under the experimental conditions, the methanol synthesis catalyst C306 in the low temperature section is the methanol production reaction rate under the K 483-513 scope to be superior to the C302 and the C301 catalyst under the same condition.
Copper based catalyst:
• CuO/ZnO/Cr2O3;
• CuO/ZnO/Al2O3;
• CuO/ZnO/Si2O3;
• CuO/ZnO/ZrO;
The role of CuO/ZnO/Al2O3 in the components: the active centre: the reduced Cu-CuO interface. ZnO: as an auxiliary agent, enhance the Cu dispersion, enhance the activity of the catalyst. Al2O3: as a carrier, at the same time prevent part of CuO reduction.
Methanol synthesis from CO2 hydrogenation
Catalyzer:
1. Copper based catalyst (Cu/ZnO/ZrO2)
• Cu0 and Cu+;
• Cu0 or Cu+;
• Cu2O and ZrO;
• Oxygen vacancies and Cu.
2. Supported catalyst
Pd/Cu/ZnO/Al2O3
Mechanism of methanol synthesis from CO2 hydrogenation over Cu/ZnO/ZrO2 catalyst:
Arena study on Cu/ZnO/ZrO2 catalyst for CO2 hydrogenation reaction mechanism, they think that ZnO/ZrO2 can be adsorbed CO2 generate formate, copper dissociative adsorption of hydrogen through the overflow effect reached Cu/ZnO and Cu/ ZrO2 interface and formate methanol synthesis.
Comparison of reaction rates on different catalysts. The calculation model has macro kinetics of catalyst, the reaction rate constant K, CO, CO2, K and the C306 values of the constants corresponding kinetic model were compared. Results: under the same temperature, the catalyst of C301 and C302k value than close, C306 is significantly larger, but less than C302. These three kinds of catalysts in the same industry under the condition of methanol production rate, the low temperature is about 483-513 in the range of K, the reaction rate of C306 catalyst for methanol formation, and has maintained a high level, this phenomenon with the Li Yan switch research results.
The reaction rate constant, the size of the most important factors of the reaction rate is respectively corresponding, and methanol synthesis rate is CO and CO2 reaction rate and root cause, therefore this phenomenon may be: the temperature is less than 513 K, the catalyst C306 KL significantly higher when the temperature exceeds 513 K. After C306, methanol formation reaction rate is less than that of C302, this may be because the C302 sharply increased.
Discussion and Conclusion
Methanol is one of the simplest chemicals, is an important chemical raw material and clean liquid fuel, widely used in organic synthesis, dyes, medicine, pesticide, automotive, defence and other fields. With the development of methanol synthesis, synthesis catalyst has been studied and improved the copperbased catalyst is a catalyst in the synthesis of low pressure, and methanol plays more important.
Conflict of Interest
The authors declare no conflict of interest.
References
- Hao AX, Yang CH, Mao CP, Wei SX, Yin Y (2013) Effect of surface modification on the catalytic performance of Cu/ZnO/Al2O3 methanol synthesis catalyst. Journal of Physical Chemistry 9: 2047-2055.
- Ray K (2014) The basic research of the new catalyst for methanol synthesis and the mathematical simulation of the reactor. East China University of Science and Technology, Shanghai, China.
- Xiao J (2015) Carbon supported CuO/ZnO catalyst for new CO_2 hydrogenation synthesis of methanol. Northeastern University, Shenyang, China.
- Guo XM (2011) Study on Synthesis of methanol copper-based catalyst by carbon dioxide hydrogenation. East China University of Science and Technology, Shanghai, China.
- Ping P (2012) Study on the process technology of largescale coal methanol. East China University of Science and Technology, Shanghai, China.
- Wei Y (2013) For non-synthetic gas products of low temperature methanol steam reforming reaction of copper zinc gallium catalyst. East China University of Science and Technology, Shanghai, China.
- Zhao YP (2013) Preparation and performance study of CuO-ZnO/TiO_2 catalyst for the synthesis of methanol from greenhouse gas CO_2. Harbin Institute of Technology, Harbin, China.
- He G (2010) Research and development status and Prospect of methanol synthesis catalyst of copper series. Chemical Engineering of Oil & Gas 39: 22-17.
- Zhuang HD, Bai SF, Liu XM, Yan ZF (2010) Cu/ZrO_2 catalyst structure and its CO_2 hydrogenation synthesis of methanol catalytic reaction performance. Journal of Fuel Chemistry 4: 462-467.
- Guo YX (2010) Research progress of copper-based methanol synthesis catalysts prepared by precipitation method. Chemical Industry and Engineering Technology 5: 42-45.
- Wei M (2013) Optimization of KATALCO51-9 process conditions for methanol synthesis catalyst. Dalian University of Technology, Dalian, China.
- Tang XB, Noritatsut S, Han YZ, Hong J, Tan Y (2014) Different modification effects of components in low temperature methanol synthesis performance of Cu-ZnO catalysts. Journal of Fuel Chemistry 6: 704-709.
- Wang GL, Li CY (2001) Synthesis of methanol from syngas and its progress in methanol synthesis. Chemical Industry 3: 42-46.
- Yang YQ, Han XM, Qeng L, Teng DD, Yuan YZ (2001) UVDR, TPD and TPR Characterization of Cu-Zn-Al-Zr Catalyst for Methanol Synthesis. Journal of Xiamen University 6: 1251-1255.
- Dong X, Zhang HB, Lin GD, Yuan YZ, Cai QR (2002) Carbon nanotubes promoted Cu-based efficient methanol synthesis catalyst. Journal of Xiamen University 2: 135-140.
- Cheng CL (2013) Study on Catalysts for methanol synthesis from carbon dioxide hydrogenation. Shanghai Normal University, Shanghai, China.
- Cheng G (2013) Carbon dioxide and copper-based catalyst for methanol synthesis of hydrogen preparation and properties. Qiqihar University, China.
- Guo YY (2013) The hydrogenation of carbon dioxide methanol synthesis catalyst Cu/ZrO_2/CNTs. South China University of Technology, Guangzhou, China.
- Han H, Wang HS, Yang XZ, Zhu BC, Fang DY (2003) Intrinsic kinetics of methanol synthesis catalyst XNC-98. Journal of East China University of Science and Technology 5: 433-440.
- Guo XJ, Chen BY, Bao GL, Li LM (2003) Study on the properties and structure of copper-based methanol synthesis catalysts prepared by different preparation methods. Natural Gas Chemical Industry 2: 9-13.
- Ma HL (2003) The study of copper-based methanol synthesis catalyst. Zhejiang University of Technology, Hangzhou, China.
- Yin YQ, Xiao TC, Su JX, Wang HT, Lu YL (2000) The copper-based methanol synthesis catalyst deactivation. Molecular Catalysis 5: 373-378.
- Yang YL, Liu ZH, Zuo Q, Wu LQ (2000) The use of methanol synthesis catalyst to summarize the experience. Natural Gas Chemical Industry 2: 37-43.
- Guo XJ, Li LM, Liu SM, Bao GL, Hou WH (2007) Effect of slurry prepared by mixing copper-based methanol synthesis catalysts and additives Al_2O_3. Journal of fuel chemistry and technology 3: 329-333.
- Chang Z., Yang YQ, Xu PP, Zhang HB, Wang SC, et al. (1995) Modified copper-based catalyst XNC208 for methanol synthesis of TPD, TPO and TPR. Journal of Xiamen University 4: 566-571.
- Zhao ZY (2006) Study on Synthesis of methanol catalyst and optimal operation of the synthetic reaction. Daqing Petroleum University, Daqing, China.
- Ci ZM (2006) For methanol synthesis from CO hydrogenation catalyst. Sichuan University, Chengdu, China.
- Zhao J (2007) Synthesis, characterization and electrocatalytic activity of the anode catalysts for direct methanol fuel cells. Zhejiang University, Hangzhou, China.
- Shang MJ (2011) Study on Synthesis of methanol catalyst by carbon dioxide hydrogenation. Dalian University of Technology, Dalian, China.
- Zheng YF, Li XN, Cen YQ, Liu HZ (2006) Catalyst composition of acid alkali alternate modern chemical effects of copper-based catalysts for methanol synthesis by precipitation. Journal of fuel chemistry and technology 3: 46-49.
- Bahruji H, Bowker M., Hutchings G, Dimitratos N, Wells P, et al. (2016) Pd/ZnO catalysts for direct CO2 hydrogenation to methanol. Journal of Catalysis 03: 17-25.
- Bonura G, Cannilla C, Frusteri L, Mezzapica A, Frusteri F (2016) DME production by CO2 hydrogenation: Key factors affecting the behaviour of CuZnZr/ferrierite catalysts. Catalysis Today 5: 57-65.
- Cai W, de la Piscina PR, Toyir J, Homs N (2014) CO2 hydrogenation to methanol over CuZnGa catalysts prepared using microwave-assisted methods. Catalysis Today 6: 193-199.
- da Silva RJ, Pimentel AF, Monteiro RS, Mota CJA (2016) Synthesis of methanol and dimethyl ether from the CO2 hydrogenation over Cu·ZnO supported on Al2O3 and Nb2O5. Journal of CO2 Utilization 15: 83-88.
- Deerattrakul V, Dittanet P, Sawangphruk M, Kongkachuichay P (2016) CO2 hydrogenation to methanol using Cu-Zn catalyst supported on reduced graphene oxide nanosheets. Journal of CO2 Utilization 16: 104-113.
- Dong X, Li F, Zhao N, Xiao F, Wang J, et al. (2016) CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts prepared by precipitation-reduction method. Applied Catalysis B: Environmental 191: 8-17.
- Dumrongbunditkul P, Witoon T, Chareonpanich M, Mungcharoen T (2016) Preparation and characterization of Co–Cu–ZrO2 nanomaterials and their catalytic activity in CO2 methanation. Ceramics International 42: 10444-10451.
- Jiang X, Koizumi N, Guo X, Song C (2015) Bimetallic Pd–Cu catalysts for selective CO2 hydrogenation to methanol. Applied Catalysis B: Environmental 170-171: 170-185.
- Kunkes EL, Studt F, Abild-Pedersen F, Schlögl R, Behrens M (2015) Hydrogenation of CO2 to methanol and CO on Cu/ZnO/Al2O3: Is there a common intermediate or not? Journal of Catalysis 328: 43-48.
- Lei H, Nie R, Wu G, Hou Z (2015) Hydrogenation of CO2 to CH3OH over Cu/ZnO catalysts with different ZnO morphology. Fuel 154: 161-166.
- Li MMJ, Zeng Z, Liao F, Hong X, Tsang SCE (2016) Enhanced CO2 hydrogenation to methanol over CuZn nanoalloy in Ga modified Cu/ZnO catalysts. Journal of Catalysis 3: 20-29.
- Marcos FCF, Assaf JM, Assaf EM (2016) Catalytic hydrogenation of CO2 into methanol and dimethyl ether over Cu-X/V-Al PILC (X=Ce and Nb) catalysts. Catalysis Today 8: 7-15.
- Ren H, Xu CH, Zhao HY, Wang YX, Liu J, et al. (2015) Methanol synthesis from CO2 hydrogenation over Cu/γ-Al2O3 catalysts modified by ZnO, ZrO2 and MgO. Journal of Industrial and Engineering Chemistry 28: 261-267.
- Sun K, Fan Z, Ye J, Yan J, Ge Q, et al. (2015) Hydrogenation of CO2 to methanol over In2O3 catalyst. Journal of CO2 Utilization 12: 1-6.
- Tahir M, Tahir B, Saidina Amin NA, Alias H (2016) Selective photocatalytic reduction of CO2 by H2O/H2 to CH4 and CH3OH over Cu-promoted In2O3/TiO2 nanocatalyst. Applied Surface Science 389: 46-55.
- Toyir J, Ramírez de la Piscina P, Homs N (2015) Ga-promoted copper-based catalysts highly selective for methanol steam reforming to hydrogen; relation with the hydrogenation of CO2 to methanol. International Journal of Hydrogen Energy 40: 11261-11266.
- Witoon T, Chalorngtham J, Dumrongbunditkul P, Chareonpanich M, Limtrakul J (2016) CO2 hydrogenation to methanol over Cu/ZrO2 catalysts: Effects of zirconia phases. Chemical Engineering Journal 293: 327-336.
- Witoon T, Kachaban N, Donphai W, Kidkhunthod P, Faungnawakij K, et al. (2016) Tuning of catalytic CO2 hydrogenation by changing composition of CuO–ZnO–ZrO2 catalysts. Energy Conversion and Management 118: 21-31.
- Xu J, Su X, Liu X, Pan X, Pei G, et al. (2016) Methanol synthesis from CO2 and H2 over Pd/ZnO/Al2O3: Catalyst structure dependence of methanol selectivity. Applied Catalysis A: General 514: 51-59.
- Zhang Y, Zhong L, Wang H, Gao P, Li X, et al. (2016) Catalytic performance of spray-dried Cu/ZnO/Al2O3/ZrO2 catalysts for slurry methanol synthesis from CO2 hydrogenation. Journal of CO2 Utilization 15: 72-82.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences